 Genetics may play a larger role in causing Lou Gehrig’s disease than previously believed, potentially accounting for more than one-third of all cases, according to one of the most comprehensive genetic studies to date of patients who suffer from the condition also known as amyotrophic lateral sclerosis, or ALS.
Genetics may play a larger role in causing Lou Gehrig’s disease than previously believed, potentially accounting for more than one-third of all cases, according to one of the most comprehensive genetic studies to date of patients who suffer from the condition also known as amyotrophic lateral sclerosis, or ALS.
The study, conducted by investigators at Cedars-Sinai and Washington University in St. Louis, also showed that patients with defects in two or more ALS-associated genes experience disease onset about 10 years earlier than patients with single-gene mutations.
“These findings shed new light on the genetic origins of ALS, especially in patients who had no prior family history of the disease,” said Robert H. Baloh, MD, PhD, director of neuromuscular medicine in the Department of Neurology and director of the ALS Program at Cedars-Sinai. Baloh is senior author of the study, published online in Annals of Neurology.
Typically, researchers classify 90 percent of ALS cases as “sporadic,” meaning they occur in patients without a family history of the disease. In their study, however, the researchers found a significant degree of genetic involvement in patients with no family history. Examining DNA from 391 individuals, they identified numerous new or very rare ALS gene mutations in such people. Added to the 10 percent of cases already known to be genetic because of family history, the study suggested that more than one-third of all ALS could be genetic in origin.
Baloh said the presence of the new and rare mutations, found among 17 genes already known to be associated with ALS, does not necessarily mean they all cause the disease. But they are considered likely suspects — especially in combination. ALS often is caused by well-known defects in single genes, but recent studies have suggested that some cases could be brought on by the simultaneous occurrence of two or more “lesser” genetic defects. In theory, each mutation alone might be tolerated without initiating disease, but in combination they exceed the threshold required for disease development.
This study strengthens that possibility: Fifteen patients — nine of whom had no previous family history of ALS — had mutations in two or more ALS-associated genes. The research also takes an important next step, showing that multiple genetic defects can influence the way disease manifests in individual patients. Those with mutations in two or more genes had onset about 10 years earlier than those with defects in only one gene.
Matthew B. Harms, MD, assistant professor of neurology at Washington University and co-corresponding author of the article, said that unknown factors still accounted for the majority of ALS cases.
“This tells us that more research is needed to identify other genes that influence ALS risk, and that ultimately, individuals may have more than one gene contributing toward developing disease,” Harms said.
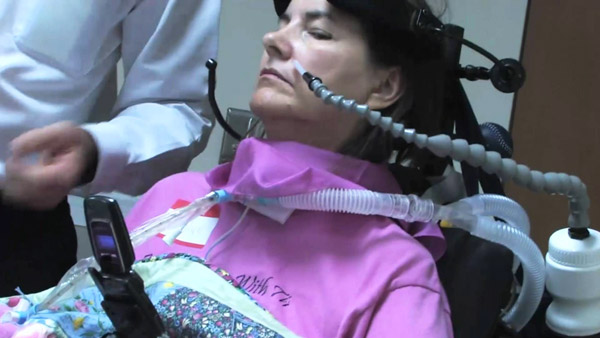
ALS is an incurable, virtually untreatable neurodegenerative disease that attacks motor neurons — nerve cells responsible for muscle function — in the brain and spinal cord. It causes progressive weakness and eventual failure of muscles throughout the body; patients typically survive three to five years after onset.
Investigators in this study used new-generation technology that quickly and efficiently determines the organizational structure of large numbers of genes. They expect this and similar research to usher in personalized medicine in ALS that will allow healthcare teams to analyze a patient’s entire genetic makeup and deliver gene-specific therapies to correct detected defects. Cedars-Sinai researchers recently conducted a disease-in-a-dish study with cells from patients with defects in a gene that commonly causes ALS. Using small segments of genetic material to target the defects, they showed that this type of gene therapy can improve neurons from patients with the disease.
These individualized-treatment studies recently received a $1.6-million boost from the ALS Association, which awarded the funds to the Cedars-Sinai Board of Governors Regenerative Medicine Institute as part of an initial distribution of money raised by the ALS Ice Bucket Challenge. With this funding, investigators will employ a specialized stem cell process to create motor neurons from a large number of patients with ALS. The study done by Cedars-Sinai Medical Center.