8. Calabar bean

Calabar bean is the seed of a leguminous plant, Physostigma venenosum, a native of tropical Africa, poisonous to humans. It derives the first part of its scientific name from a curious beak-like appendage at the end of the stigma, in the centre of the flower; this appendage, though solid, was supposed to be hollow.
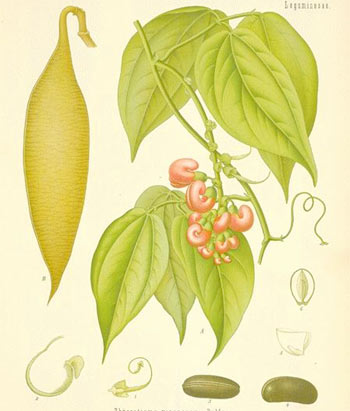
The plant is a large, herbaceous, climbing perennial, with the stem woody at the base, up to 2 inches (5 cm) in diameter; it has a habit like the scarlet runner, and attains a height of about 50 feet (15 m). The flowers, resting on axillary peduncles, are large, about an inch long, grouped in pendulous, fascicled racemes pale-pink or purplish, and beautifully veined. The seed pods, which contain two or three seeds or beans, are 6 or 7 inches (15 or 18 cm) in length; and the beans are about the size of an ordinary horse bean but much thicker, with a deep chocolate-brown color.
Toxicology
Calabar bean contains physostigmine, a reversible cholinesterase inhibitor alkaloid. The alkaloid physostigmine acts in effect like nerve gas, disrupting communication between the nerves and muscles, and resulting in copius salivation, seizures, loss of control over the bladder and bowels, and eventually loss of control over the respiratory system, causing death by asphyxiation.
The main antidote to Calabar bean poisoning is atropine, which may often succeed; and the other measures are those usually employed to stimulate the circulation and respiration. Unfortunately, the antagonism between physostigmine and atropine is not perfect, and Sir Thomas Richard Fraser has shown that in such cases there comes a time when, if the action of the two drugs is summated, death results sooner than from either alone. Thus atropine will save life if three and a half times the fatal dose of physostigmine has been taken, but will hasten the end if four or more times the fatal dose has been ingested
Calabar bean is a plant. The seed is extremely poisonous and is also used to make medicine. Historically, African tribes used calabar bean, the “ordeal bean,” to identify witches and people possessed by evil spirits. They believed that people who were able to eat the bean and live were innocent. Subjects of the “ordeal” could increase their chance of survival by not chewing the bean but, instead, swallowing it whole. Chewing releases the poisons in the bean. Ritual uses continue in Africa despite being outlawed.
As medicine, Calabar bean is used for eye problems, constipation, epilepsy, cholera, and tetanus. Calabar bean is a source of the prescription drug physostigmine (Isopto Eserine, Antilirium).
Calabar bean contains a chemical that affects signals between muscles and nerves. This chemical affects many parts of the body.
Calabar bean is unsafe. It is extremely toxic.
Calabar bean can cause excessive saliva and sweating, reduced eye pupil size, nausea, vomiting, diarrhea, irregular heartbeat, blood pressure changes, confusion, seizures, coma, severe muscle weakness, paralysis, severe breathing problems, and death.
Special Precautions & Warnings:
While calabar bean isn’t safe for anyone, some people are at even greater risk for serious side effects. Be especially careful to avoid calabar bean if:
- You are pregnant or breast-feeding.
- You have Parkinson’s disease.
- You have heart disease or slow heartbeat.
- You have asthma.
- You have diabetes.
- You have poor blood circulation leading to tissue death (gangrene).
- You have blockage of the intestinal tract.
It is a creeping plant up to 6 m long and has 15-cm long fruits. Its seeds are dark brown, kidney-shaped and very similar to ordinary beans, which is why they cause accidents very often. They are extremely poisonous–one seed is enough to kill a person.
Local people are familiar with the toxic effect of this plant. They used it in the public trials to challenge the innocence of the accused by administering a poison prepared from Calabar bean. Surviving this poisoning was considered a proof of innocence but it was an extremely rare occasion.
Its medical significance was discovered in 1855, who received some seeds from an African missioner to facilitate his research. Nowadays, the seeds of Physostigma venenosum are used as a source of the valuable alkaloid physostigmin. Its application is limited to ophthalmology and toxicology. It is also effective in cases of diseases of the peripheral nervous system, muscle and bowel diseases but its usage is now limited because of its toxicity.
 Mid-19th century European visitors to Old Calabar, an eastern province of Nigeria, could not avoid becoming aware of native belief in the power of the seeds of a local plant to determine whether individuals were innocent or guilty of some serious misdemeanour. The seeds were those of a previously unknown legume and soon referred to as the ordeal bean of Old Calabar. Their administration was known locally as ‘chop nut’. Missionaries who arrived in Calabar in 1846 estimated that chop nut caused some 120 deaths annually and documented the course of poisoning. The latter information and samples of the beans rapidly found their way to Scotland, the home of the missionaries’ parent church, explaining why the early toxicology of physostigmine, quantitatively the most important of three active alkaloids in the beans, has such strong Scottish, predominantly Edinburgh, associations. However, it was 1855 before the first of many medical scientists, Robert Christison, a toxicologist of repute, investigated the effects of the beans to the extent of eating part of one himself and documenting the moderate, if not severe, consequences. A further 6 years were to pass before Balfour’s comprehensive botanical description of the bean plant appeared. It was he who named it Physostigma venenosum. It was not so long until the next event, one that sparked more intensive and international interest in the beans. In 1863 a young Edinburgh ophthalmologist, Argyll Robertson, published a paper announcing the arrival of the first agent that constricted the pupil of the eye. The drug was an extract of Calabar beans and Argyll Robertson openly admitted that he had been alerted to its unusual property by his physician friend, Thomas Fraser.
Mid-19th century European visitors to Old Calabar, an eastern province of Nigeria, could not avoid becoming aware of native belief in the power of the seeds of a local plant to determine whether individuals were innocent or guilty of some serious misdemeanour. The seeds were those of a previously unknown legume and soon referred to as the ordeal bean of Old Calabar. Their administration was known locally as ‘chop nut’. Missionaries who arrived in Calabar in 1846 estimated that chop nut caused some 120 deaths annually and documented the course of poisoning. The latter information and samples of the beans rapidly found their way to Scotland, the home of the missionaries’ parent church, explaining why the early toxicology of physostigmine, quantitatively the most important of three active alkaloids in the beans, has such strong Scottish, predominantly Edinburgh, associations. However, it was 1855 before the first of many medical scientists, Robert Christison, a toxicologist of repute, investigated the effects of the beans to the extent of eating part of one himself and documenting the moderate, if not severe, consequences. A further 6 years were to pass before Balfour’s comprehensive botanical description of the bean plant appeared. It was he who named it Physostigma venenosum. It was not so long until the next event, one that sparked more intensive and international interest in the beans. In 1863 a young Edinburgh ophthalmologist, Argyll Robertson, published a paper announcing the arrival of the first agent that constricted the pupil of the eye. The drug was an extract of Calabar beans and Argyll Robertson openly admitted that he had been alerted to its unusual property by his physician friend, Thomas Fraser.
A minor flood of contributions on the ophthalmic uses of bean extracts followed in the medical press in the next few months; those on their systemic toxicity were fewer. Fraser’s MD thesis, submitted to the University of Edinburgh in 1862 and clearly pre-dating Argyll Robertson’s involvement with the beans, became generally available a few weeks after the appearance of Argyll Robertson’s paper and was the first to address in detail the features of systemic administration of extracts of the beans.
A major problem facing all early researchers of the beans was that of deciding how best to extract their active principle, a task made all the more difficult because bioassays were the only means of determining if the toxin was being tracked. The stability of extracts was an inevitable issue and the active principle finally became known as physostigma or physostigmine, after the botanical name of the parent plant. The features of physostigmine toxicity were soon exhaustively documented, both in animals and humans. How they were mediated was another matter altogether.
Fraser maintained that muscular paralysis, the cardinal feature, was the result of depression of the spinal cord and was generally, but far from unanimously, supported. Of those who had reservations, Harley was the most prominent. He concluded that paralysis was secondary to effects on the motor nerve endings and, in so doing, came nearest to present-day knowledge at a time when acetylcholine, cholinesterases and cholinesterase inhibitors were not even imagined. Differences of opinion on the mode of action of the beans were to be expected and it is hardly surprising that they were not resolved.
No standard formulation of physostigmine was available so the potency of those used would have varied from one investigator to another, the range of animals experimented upon was large while the number used by any researcher was commonly in single figures, more readily available cold-blooded creatures seemed less sensitive to physostigmine toxicity than warm-blooded ones and only Fraser determinedly pursued an answer; in general, the others made one foray into bean research then turned their attentions elsewhere. The same problems would beset other aspects of bean research. While Fraser did not get as close to the mode of action of physostigmine as Harley, he reigns supreme when it comes to antagonism between physostigmine and atropine.
By this time, the 1870s had dawned and although the concept of antagonism between therapeutic agents was not new, it had little, if any, reliable scientific foundation. This was about to change; antagonism was becoming exciting and rational. Fraser’s firm belief that physostigmine and atropine were mutually antagonistic at a physiological level was contrary to the conventional wisdom of his contemporaries. This alone would earn him a place in history but his contribution goes much, much further. Unlike any other at the time, he investigated it with scientific rigour, experimenting on only one species, ensuring as best he could the animals were the same weight, adjusting the doses of drugs he gave them for bodyweight, determining the minimum lethal dose of each drug before assessing their antagonistic effects, adopting a single, incontrovertible endpoint for efficacy and carrying out sufficient numbers of experiments to appear convincing in a later era where the statistical power of studies is all-important.
To crown it all, he presented his results graphically. Fraser never claimed to have discovered the antagonism between physostigmine and atropine. Bartholow in 1873 did, based on work done in 1869. But his data hardly justify it. If anyone can reasonably claim this particular scientific crown it is an ophthalmologist, Niemetschek, working in Prague in 1864. His colleague in the same discipline, Kleinwächter, was faced with treating a young man with atropine intoxication. Knowing of the contrary actions of the two drugs on the pupil, Niemetschek suggested that Calabar bean extract might be useful. Kleinwächter had the courage to take the advice and his patient improved dramatically. Clearly, this evidence is nothing more than anecdotal, but the ophthalmologists were correct and, to the present day, physostigmine has had an intermittent role in the management of anticholinergic poisoning.
The converse, giving atropine to treat poisoning with cholinesterase inhibitors, of which physostigmine was the first, has endured more consistently and remains standard practice today. It is salutary to realise that the doses and dosage frequency of atropine together with the endpoints that define they are adequate were formulated by Fraser and others a century and a half ago.
Disclaimer
The Content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition.